Shannon P. Finnegan, Amael Hinojo, Sarah Monod, William A. Wall, Peter Olsen, Maximilian L. Allen
Journal of Wildlife Biology : https://nsojournals.onlinelibrary.wiley.com/doi/10.1002/wlb3.01360
Abstract
One of the most difficult challenges for wildlife managers is reliably estimating wildlife populations. Camera traps combined with spatial capture–recapture (SCR) models are a popular tool for population estimation. They have limitations, however, including long data processing times. Drones with thermal imagery are an emerging tool for estimating wildlife populations, but how they compare to other methods remain poorly studied. We compared the use of camera traps and SCR models to drone surveys for estimating population densities of Sitka black-tailed deer Odocoileus hemionus sitkensis on Afognak Island, Alaska. We deployed 26 camera traps from 1 September until 6 October 2022 and individually identified males using antler characteristics, for the SCR model. At the same site we conducted three drone surveys between October and December 2022, identified sex composition and obtained deer counts. The estimated density from the SCR model was 3.7 males ± 0.8 (SE) km–2, and 14.1 ± 3.1 adults km–2 of clear-cut forest. Results from the drone survey produced similar estimates with 2.1 ± 0.9 males km–2 and 13.4 ± 1.6 adults km–2. The similarity in estimates suggests that both methods converged on an accurate representation of the population in this habitat, but these methods diverge in levels of sampling effort, duration, and financial cost. Camera traps offer further insights on behavior and home-range size but require longer data processing times, can be subject to malfunctions, and are difficult to deploy and maintain in remote areas. Drones are subject to legal restrictions, have difficulty in closed canopy habitat and can be initially costly, but they provide results faster and require less data analysis. Camera traps and drones are useful for determining population dynamics but are subject to their limitations. Wildlife managers should make survey decisions based on their specific goals, habitat type, focal species ecology and financial limitations.
Introduction
Effective wildlife management relies on regular population monitoring, including accurate estimates of species abundance over time (Ransom et al. 2012). For ungulates, population estimation is a requisite for setting adequate hunting quotas, measuring the impacts of conservation actions, and tracking population responses to management programs (Witczuk et al. 2018, Hinojo et al. 2022). However, many species are widely dispersed and occur in remote areas, making assessments of population sizes and trends difficult (Rich et al. 2019, Beaver et al. 2020). This can be further complicated when remote habitats experience large-scale landscape changes (e.g. anthropogenic activities, fire), resulting in unknown effects on wildlife populations. Without quantitative estimates of population sizes over time, harvest quotas set by management bodies may not be reflective of changing population sizes (Cobb 2014).
Numerous methods have been applied for population estimates of deer species, including spotlight surveys (DeYoung 2011), harvest data reconstruction (Millspaugh et al. 2009), pellet counts (Goode et al. 2014) and track surveys (Fritzen et al. 1995). However, these are labor intensive, not ideal for remote areas, and subject to bias and imprecise results (Beaver et al. 2014, Forsyth et al. 2022). Camera trapping combined with new analytical frameworks have become a more favored tool for deer population estimation (Macaulay et al. 2020, Forsyth et al. 2022, Hinojo et al. 2022). When individuals are recognizable (i.e. antler formation, body scars, ear tags, etc.), density can be derived using spatial capture–recapture (SCR) models (Macaulay et al. 2020). Male only identification has also been used to derive estimates using the Jacobson index (Weckel et al. 2011). Recently models have been developed which derive from SCR but incorporate the spatial patterns of detections to determine individual identities of unmarked animals (Sun et al. 2022). These spatial count (SC) and spatial partial identity models (SPIM) are subject to limitations with sample design and may produce biased estimates of population size (Sun et al. 2022). While camera trapping methods are rapidly advancing, particularly for use on unmarked populations, they are still labor intensive, particularly with data processing (Swanson et al. 2015) – although machine learning can reduce this workload (Tabak et al. 2019). Camera trapping can also be difficult when working in remote areas, since it may require long periods of time to collect data and is sometimes limited by equipment malfunctions.
New technological developments offer the potential to create innovative and cost-effective tools to estimate wildlife population sizes. Drones have recently become a popular technology used in studies of natural resources. Drones fitted with thermal imaging cameras are advantageous due to their comparable, and sometimes increased, animal detection rates compared to other survey techniques (Beaver et al. 2020, Zabel et al. 2023). Nevertheless, detection probability of aerial counts declines with increasing tree cover (Franke et al. 2012, Forsyth et al. 2022). Drones may offer greater access to remote areas and have lower operational costs (Baldwin et al. 2023). They may also be more efficient for data processing (Baldwin et al. 2023), particularly if surveys are recorded in a video format which can be watched by observers relatively quickly compared to the long image tagging times required for camera trap data (Baldwin et al. 2023). However, how well drone population estimates compare to the more commonly used camera trapping survey techniques remains understudied.
Sitka black-tailed deer Odocoileus hemionus sitkensis were first introduced to the Kodiak Archipelago, Alaska, between 1924 and 1934. The population has since greatly expanded in both range and size, possibly exceeding 100 000 animals in the mid- to late-1980s (Smith 1989). The population appears subject to drastic fluctuations in size, likely driven by the severity of winter weather. Deer are a resource of high importance, both economically and ecologically, across the Kodiak Archipelago. They are the most important source of terrestrial subsistence meat for Kodiak residents, even surpassing marine mammal protein, and are an eligible species for intensive management (IM) by the Alaska Department of Fish and Game on Kodiak (J. Keating – Alaska Department of Fish and Game, pers. comm. 2023). Sitka black-tailed deer are also likely an important food source for brown bears across the archipelago (Van Daele et al. 2013, Finnegan et al. 2023) and potentially play a keystone ecological role on plant diversity through the effects of their browsing (Cobb 2014). Despite the importance of this resource, very little is known about their population abundance.
Our objective was to compare two methods for estimating free ranging Sitka black-tailed deer population sizes on a timber clear-cut site on Afognak Island in the Kodiak Archipelago. Using standard camera trapping techniques, we calculated an adult and male deer density estimate and sex ratios using spatial capture–recapture (SCR) models. At the same site we estimated densities and sex ratios from aerial thermal imaging drone surveys. We compared the feasibility, accuracy, and cost estimates for both methods to aid future wildlife population management and to validate new tools for advancing wildlife population estimations in remote areas.
Material and methods
Study site
Afognak Island (58°19ʹ40.4ʺN, 152°38ʹ29.4ʺW) (1809 km2) is situated in the Kodiak Archipelago, Alaska, USA, 5 km north of Kodiak Island and separated by a 1.5 km wide ocean strait (Fig. 1). The island contains rolling mountains with elevations up to 739 m a.s.l. Average annual rainfall and snowfall for the archipelago are 198 and 189 cm, respectively. The archipelago has a subarctic maritime climate with average annual high and low temperatures of 7.9 and 1.9°C, respectively (Finnegan et al. 2021a, b2021b). Sitka spruce Picea stichensis is the dominant tree species on Afognak, while devil’s club Oplopanax horridus, blueberry Vaccinium ovalifolium, salmonberry Rubus spectabilis, and willow (Salix spp.) are dominant understory species. The island was commercially logged during the 1930s and more intensively since 1979 until present day. Our 2.36 km2 study site occurred within a clear-cut timber unit less than 10 years post-harvest. Post logging, numerous understory species became abundant at the site, which has resulted in productive foraging habitat for deer. The island is primarily owned by Alaska Native Claims Settlement Act (ANCSA) corporations (64%) and state (27%) and federal (9%) governments. Roosevelt elk Cervus canadensis roosevelti occur on the island since introduction to Afognak in 1929, while brown bears are native to the Kodiak Archipelago and occur across Afognak.
Figure 1
Map of 2.36 km2 study site on Afognak Island, Alaska, with camera trap locations,drone survey route, and photograph of clear-cut timber habitat type present in the study area.
Camera trapping
We placed 26 camera traps (Browning Strike Force HD- 20MP) at un-baited sites, spaced on average 400 m apart along inactive logging roads. Roads here are heavily used by deer as the adjacent clear-cut areas are comprised heavily of slash piles, making movement more difficult (Finnegan et al. unpubl.). Roads and trails have been used for camera locations in previous studies focused on deer densities in Central America (Soria-Díaz and Monroy-Vilchis 2015). Cameras were fixed to poles or naturally occurring stumps at an average height of approximately 100 cm off the ground. We removed any vegetation in front of the camera view shed to reduce false triggers. Cameras were active from 1 September until 6 October 2022 and were set to operate 24 hours a day with a three photo capture series upon trigger, and a three second delay between trigger events.
SCR models
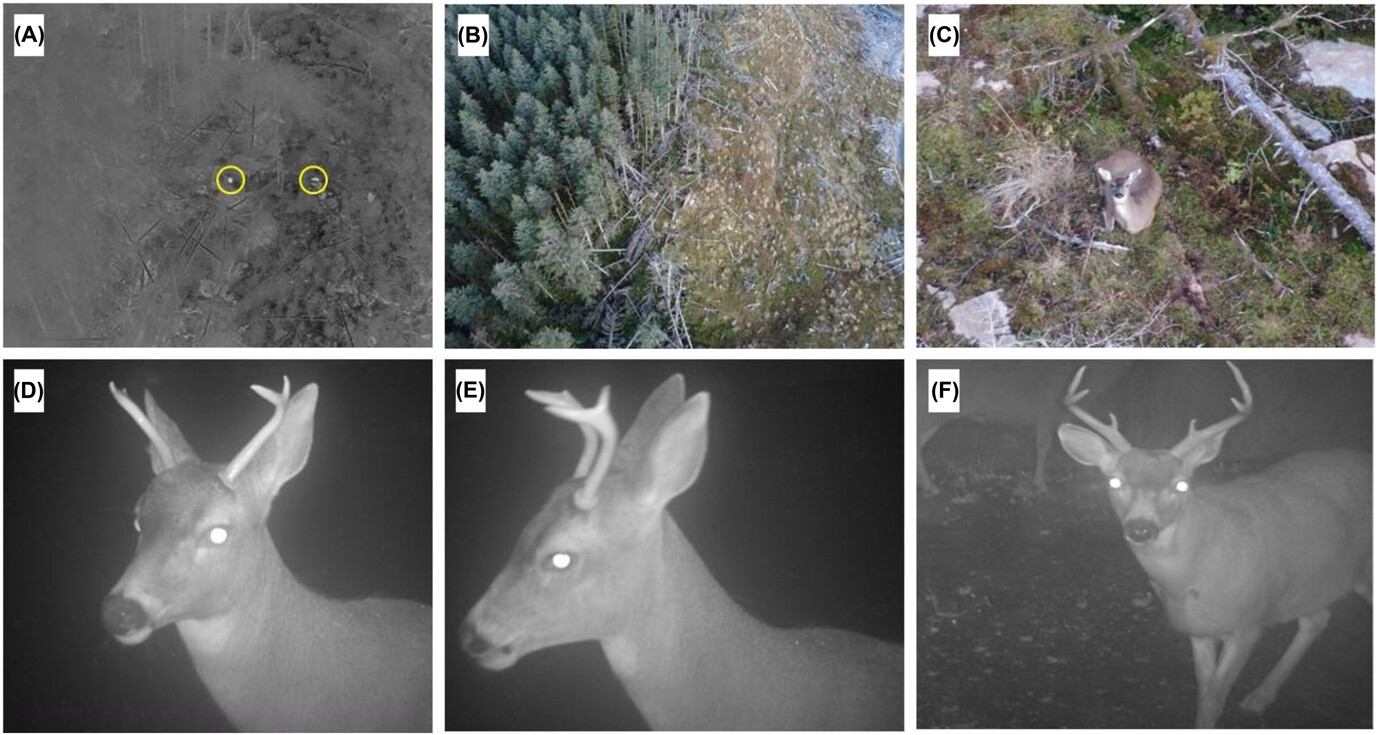
Within the SCR modelling framework, focal animal detections should be independent from each other, therefore we used a six-minute cutoff between deer capture events at a given camera based on prior information on deer activity at camera sites (Macaulay et al. 2020). For each encounter, we identified the sex of individuals when possible (bucks, does, does with fawns) and recorded group size, date, time, and camera location. One observer individually identified bucks manually based on antler characteristics (e.g. shape, length, number of points, brow point morphology) and other natural markings such as body scars (Hinojo et al. 2022, Fig. 2). These identifications were then confirmed by a second independent observer to reduce potential observer bias. We attempted to identify does where possible with natural markings (Macaulay et al. 2020). For both the camera trapping and drone surveys we determined the sex ratio (buck:doe) as the number of independent encounters of bucks divided by the sum of independent encounters of does and bucks (Hinojo et al. 2022).

Figure 2
Panels consisting of (A) thermal image taken from a drone survey showing heat signatures from a doe and fawn, (B) the regular image taken by the drone and (C) the zoomed in image of a bedded down doe. Photos were taken at an altitude of 91 meters above ground level. Image panels (D) and (E) display the same individual male deer (M14) taken from the camera trap survey, while panel (F) display a different individual male (M10) on Afognak Island, Alaska.
We analysed camera trap data using spatially explicit photographic capture–recapture models (SCR) following the methodology described to estimate roe deer densities in Hinojo et al. (2022). SCR is a set of methods for modelling animal capture–recapture data collected with an array of detectors (Efford 2020), in our case camera traps. Animal density is directly estimated using information on capture histories in combination with spatial locations of captures (Efford 2020). We used the ‘secr’ package (Efford 2020) in program R (www.r-project.org) and the input data of individual male Sitka black-tailed deer encounters (i.e. site number, date, time of the encounter, male individual identification), the location of the camera sites, and camera deployment details including dates when cameras were active. We defined a sampling occasion as a time frame of 24 hours starting at noon, which results in 32 sampling occasions.
To obtain an estimate for the adult deer density we followed the protocols described by Hinojo et al (2022). We divided the estimated male density resulting from the SCR analysis by the estimate of the sex ratio (i.e. the number of independent encounters of males divided by the number of independent encounters of females and males). In SCR analysis, a buffer width that is too narrow may inflate density estimates; therefore, it is important to determine the buffer width beyond which the density estimates start to stabilize. We created a simple plot of density estimates based on a series of null models integrating the sampling effort (Hinojo et al. 2022), which showed that SCR density estimates decreased rapidly with increasing buffer width and stabilized when the buffer width was 600 m (Supporting information); hence, we retained this width in the subsequent analysis.
In large samples, the sampling distribution of the maximum likelihood estimates of the male density tends towards a normal distribution, with mean equal to maximum likelihood estimation (MLE) and standard deviation equal to the standard error. To get an assessment of the precision of the adult density estimate, we used a parametric bootstrap. We drew 1 random number from the male density (x¯ = MLE [density], SD = SE [density]) for each draw from the sampling distribution of the corresponding sex ratio estimate. We then combined the 2 values to obtain 1 draw from the sampling distribution of the adult density. We repeated this procedure 1 million times to get an estimate of the precision of the adult deer density (Hinojo et al. 2022).
| Method | Buck:Doe ratio | Male density (km2) | Adult density (km2) | Minimum no. of bucks | g0 | σ (m) | Home range size (km2) | Data collection time | Data processing and analysis time |
|---|---|---|---|---|---|---|---|---|---|
| SCR | 1:2.6 | 3.7 (± 0.8) | 14.1 (±3.1) | 23 | 0.04 (0.006) | 343.6 (30.1) | 2.2 | 12 | 47 |
| Drone | 1:2.5 | 2.1 (± 0.9) | 13.4 (±1.6) | 9 | ~ | ~ | ~ | 6 | 6 |
Drone survey
We attempted four replicate dawn survey flights over the study area between October and December of 2022. Low ambient temperatures during this time of year, reduced vegetation, and early morning flights provided the best opportunities to easily detect deer thermal signatures from the air. We used a DJI Matrice 300 RTK (real-time kinematic) quadcopter drone equipped with a Zenmuse H20 radiometric thermal camera. This camera has a 20MP zoom, and a 12MP wide camera lens, with 1200 m integrated laser rangefinder. Flights had a preprogrammed route (Fig. 1) prior to departure and were operated by commercially licensed drone pilot (part 107 license for USA). We flew all surveys at 91 m above ground level at an average speed of 19 mph with 0% overlap between flight transects. During each flight the drone continuously recorded both thermal and video footage. Flights were automated, but once a thermal signature was identified by the pilot in command, the flight mission was paused and the zoom camera was used to identify the animal and determine sex when possible. After an animal was identified the flight mission was resumed. Flight times were on average one hour in length, depending on the number of animals counted, and required one battery change mid survey (average flight times with one battery set were 45 min). Post-survey the video footage was reviewed on a computer and the location, sex and number of individual deer was added to the database. We used an average count from all completed surveys to determine a density estimate by dividing the mean number of males observed by the survey area (male density), and by dividing the mean number of adult deer observed by the survey area (adult density). We calculated the standard error between all completed surveys for each deer cohort (male, female, fawn, unknown sex; Table 2).
| Males | Females | Unknown sex | Adults | Fawns | |
|---|---|---|---|---|---|
| 6 Oct 2022 | 9 | 21 | 6 | 36 | 6 |
| 19 Oct 2022 | 5 | 21 | 9 | 35 | 6 |
| 2 Dec 2022 | 1 | 13 | 10 | 24 | 2 |
| Mean | 5 | 18.3 | 8.3 | 31.7 | 4.7 |
| SE (±) | 2.3 | 2.7 | 1.2 | 3.8 | 1.3 |
Results
We recorded 600 independent encounters of Sitka black-tailed deer during 832 (32 nights × 26 camera traps) total camera nights. Among the independent encounters where age and sex could be identified, most consisted of single adult and sub-adult does (54.6%) and single adult and sub-adult bucks (25.8%), with the remaining 19.6% being does with a fawn. We identified 23 male individuals. Sex ratio estimates were skewed towards females (∼2.6; Table 1), which did not have enough natural marks to allow individual identification. Consequently, it was not possible to conduct density estimation with SCR for does. Model selection revealed substantial evidence for the site transient response (MK, Efford 2020). The estimated density from this model amounted to 3.7 males ± 0.8 (SE) males km–2 of clear-cut forest, with baseline encounter probability (g0) equal to 0.04 ± 0.006 (Table 1). The movement parameter (σ) of males was 343.6 ± 30.1 m, translating into a 95% home range radius of 841 m and a home range size of 2.2 km2 (Table 1).
We conducted three complete drone surveys and one partial survey between October and December 2022. An additional survey was attempted on 29 November 2022, however, was unable to be completed due to poor weather conditions and thus was not included. The flight with the highest total deer count (42 individuals) was flown on 6 Oct 2022 at 8:30 (Table 2, Fig. 3). Deer thermal signatures were easily distinguished from the background features (Fig. 2A). The surveys appeared to result in minimal disturbance to natural behaviors, as bedded animals never got up to flee (Fig. 2C), and foraging animals continued their feeding behavior. We calculated the mean and standard error for each deer cohort across three completed surveys (Table 2) and derived a male density of 2.1 (±0.9) km–2 and an adult density of 13.4 (±1.6) km–2. The buck:doe ratio determined for the drone survey was also skewed towards females at 1:2.5.

Figure 3
Map displaying drone flight path and records of Sitka black-tailed deer observed during a thermal imaging survey on the 6 October 2022, on Afognak Island, Alaska.
Discussion
This study provided the first density estimates of Sitka black-tailed deer on Afognak Island. Our density estimates (14.1 [camera traps]–13.4 [drone] deer km–2) were substantially higher than previous estimates obtained using aerial mark–recapture distance sampling on open tundra habitat in southern Kodiak Island (0.53–0.83 deer km–2) (Cobb 2014). These differences in deer densities are likely due to several factors. Firstly, fixed wing aerial surveys may undercount deer due to difficulty in detection and observer bias (Urbanek et al. 2012), while distance sampling has been criticized in its ability to obtain accurate population estimates (Melville et al. 2008). However, the differences between these studies are most likely a reflection of suitable habitat. The combination of clear-cut, intermediate regrowth and old growth timber stands likely provide higher quality forage and thermal cover which can support higher deer densities than tundra habitat (Wallmo and Schoen 1980, Hughes and Fahey 1991). Our density estimates were higher, but more comparable to previous estimates reported for unmanaged forest habitats (12 deer km–2) and logged forests (7–10 deer km–2) of southeast Alaska (Brinkman et al. 2011). However, they were substantially lower than density estimates obtained using traditional pellet sampling in other parts of southeast Alaska(35–100 deer km-2) (Kirchhoff 2018). Deer on the Kodiak Archipelago are subject to drastic fluctuations in population sizes, which have been largely attributed to winter severity (Cobb 2014). It is therefore important for wildlife managers to track population oscillations over time to implement harvest strategies that can reflect these changes (Svoboda and Crye July 2016–June 2021). Additionally, knowledge on population dynamics is important in areas with large scale landscape changes (e.g. timber harvest) to aid in habitat management decisions that can maintain or bolster wildlife populations, particularly those important for subsistence meat harvest (Titus et al. 2009).
We successfully applied drones with thermal imaging technology to survey a free roaming population of Sitka black-tailed deer and compared this approach to the more frequently used method of using camera traps paired with SCR models (Brommer et al. 2021, Hinojo et al. 2022). We found that both methodologies yielded similar density estimates, suggesting that they converged on an accurate seasonal representation of the population in this habitat. Moreover, for the first time, we compared sex-ratios obtained by two different approaches. We obtained similar results (1:2.5 and 1:2.6 for drones and SCR respectfully) which validates the use of drones and parametric bootstrap in camera trap survey to infer sex-ratio. However, these methods diverge in levels of sampling effort, duration required, and financial cost, all of which are important considerations for wildlife managers with limited resources to consider when implementing wildlife surveys (McMahon et al. 2021).
Camera traps and drones have proved to be suitable non-invasive tools for estimating ungulate population densities (Curtis et al. 2009, Macaulay et al. 2020, McMahon et al. 2021), but notably differ in the amount of time required to collect and analyze data. Camera traps require a minimum amount of trapping nights to hold significant statistical rigor (Rovero et al. 2013), while SCR models often assume population closure of the study area, an assumption that can be violated for large free roaming mammals (Dupont et al. 2019). Deploying and maintaining camera trap grids can be challenging when working in remote areas. This can further be hampered by camera failures, including wild animals damaging and altering camera shed views (Rovero et al. 2013). In comparison, drones require less hours to collect data, with often only a few surveys required over the desired study period (De Kock et al. 2021, McMahon et al. 2021, Baldwin et al. 2023). In the case of this study, both survey techniques required travel by fixed wing aircraft to the remote study area, followed by traversing logging roads in a vehicle. On average 6–7 hours of driving and camera deployment were required to begin collecting data for the SCR models. In comparison, travel to the drone launch site and completion of one survey took on average less than two hours, therefore ~ 6 hours for three flights. Data from drone surveys was also immediately available to wildlife managers (Zabel et al. 2023), whereas camera traps required a minimum number of trap nights (i.e. number of cameras × number of nights: > 60; Rovero et al. 2013) to gather meaningful data. Replicate drone surveys could be completed in a shorter period depending on the goals of the study. For instance, multiple surveys may be completed within one month rather than multiple months, similar to how aerial fixed wing surveys are conducted to estimate brown bear population sizes on Kodiak Island (Van Daele and Barnes 2010). However, it is important to consider that population densities may change in different seasons as food availability can increase or decrease and winter weather may result in open habitats being less favorable (Beier and McCullough 1990). These factors may also have attributed to the lower deer numbers recorded in December versus October in this study. Both the SCR and drone surveys were completed in the fall/early winter, and therefore likely only reflect the population density for this habitat type and season. It is important to consider that seasonality, habitat quality and food abundance would likely lead to density increases or decreases at different times of the year (Beier and McCullough 1990).
Financially drones were more expensive in this study, with the inclusion of a thermal camera the drone ranged in cost from $23 000–26 000 (depending on number of additional batteries purchased). The cost of equipment for a 26-camera trap grid was approximately $4000. However, the time and personnel required to collect and analyze data from drone surveys (approx. 6 hours) was less compared to camera trap data (approx. 47 hours). Therefore, depending on employee salary requirements, drones may be a more cost-effective methodology (Howell et al. 2021). In a similar study, Hinojo et al. (2022) estimated the cost of a 25-camera trap grid from deployment to completion of data analysis, including Swiss salary considerations, at $23 336. Although this cost can be reduced by using 20 camera traps for 20 days still obtaining reliable results. If we were to consider similar expenses for our study, camera traps remain less expensive, but not by a large margin. However, the expenses for the camera trap survey involve the material and the salaries based on Swiss rates, while the expenses for the drones only consider the cost of material. Recent advancements in drone technologies mean thermal capable drones are more commercially available at lower costs. Current models of DJI drones with thermal cameras are available at costs between $4000–6000, making them more affordable for wildlife managers and more competitive with traditional camera trap methods.
Despite the longer data processing time, camera traps do yield some benefits over drones. Most notably, drones can struggle to identify thermal signatures of animals in closed canopy habitat (Potvin and Breton 2005). When thermal signatures can be identified, it can still be difficult to determine sex due to tree branches obscuring the drone camera field of view. Camera traps do not have this limitation, and additionally individual identification of animals with unique markings or body characteristics is possible (Macaulay et al. 2020). In the case of surveys conducted in forested habitats or when individual identification is needed, camera traps may be the best compromise to get reliable wildlife population estimates. Within the SCR modeling framework, it is also possible to estimate home range sizes of the focal species (Hinojo et al. 2022), as was demonstrated in this study, and this is not possible with drone surveys. Additionally, camera traps can provide insights into animal behaviors, such as activity patterns (Finnegan et al. 2021a, b2021b), rut timings (Jarnemo et al. 2017) and herd composition (Ikeda et al. 2013), information often valuable to wildlife managers. Camera traps are less sensitive to weather limitations compared to drone surveys, which can be hampered by high ambient temperatures, strong winds and rain (Duffy et al. 2018). However, camera traps can be subject to malfunctions in the field, sometimes temperature driven, which depending on the amount of equipment failures could seriously hinder the ability to infer population density information (Swann et al. 2011). Arguably the greatest setback of utilizing drones for surveying wildlife over large areas is the limitation of identifying thermal signature in closed canopy habitat, battery life and the federal regulations prohibiting flying drones beyond the visual line of sight of the pilot (McMahon et al. 2021). However, these drawbacks can be minimized using multiple battery sets, pilot control hand off capabilities mid-flight and through visual line of sight waivers approved by the federal aviation administration.
Conclusions
We demonstrated that drones with thermal imaging capabilities and camera trap estimates can provide a non-invasive and potentially accurate tool for determining wild ungulate population densities and sex-ratios. Both camera traps and drones can be useful for determining population dynamics, however each method has its benefits and limitations (Baldwin et al. 2023). Ultimately wildlife managers should make survey decisions based on their specific goals, the habitat type they wish to survey (i.e. dense forest versus open canopy), ecology of focal species, and financial limitations (McMahon et al. 2021, Baldwin et al. 2023). Future research comparing drones and camera traps would benefit by surveying a known marked sample of animals within a wild population, (e.g. animals fitted with ear tags or global positioning system collars). Surveys of known marked animals would help develop a sightability index for drones across different habitats, and further provide insights into the comparability of camera traps versus drones. This study provides two valuable and cost-efficient approaches to deer population estimates and highlights the feasibility of using new drone technologies to aid in wildlife management, particularly in grassland and logged forest habitats.
Acknowledgements
– We thank AJ Lullo and Bethany Warner of the University of Illinois for their assistance with image tagging. We extend thanks to Nathan Svoboda and Bill Dunker at the Alaska Department of Fish and Game, Kodiak, for manuscript revision and project support.
Funding
– We thank the National Resources Conservation Service (NRCS) for supporting this project through the conservation innovation grant.
Author contributions
Shannon P. Finnegan: Conceptualization (lead); Data curation (lead); Formal analysis (equal); Investigation (lead); Methodology (lead); Project administration (lead); Supervision (lead); Writing – original draft (lead); Writing – review and editing (equal). Amael Hinojo: Formal analysis (equal); Investigation (equal); Methodology (equal); Validation (equal); Writing – original draft (supporting); Writing – review and editing (equal). Sarah Monod: Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Validation (supporting); Writing – original draft (supporting); Writing – review and editing (equal). William A. Wall: Conceptualization (equal); Writing – review and editing (supporting). Peter Olsen: Conceptualization (supporting); Funding acquisition (equal). Maximilian L. Allen: Conceptualization (equal); Investigation (supporting); Methodology (equal); Supervision (supporting); Writing – original draft (supporting); Writing – review and editing (equal).